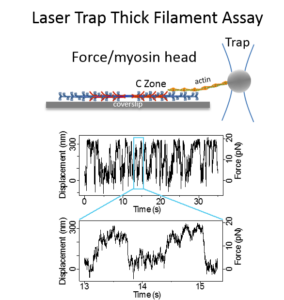
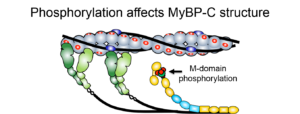
RESEARCH INTERESTSHeart and Skeletal Muscle Diseases Associated with cMyBP-C MutationsSingle point and deletion mutations in cMyBP-C have been identified as the primary cause of sudden death in humans afflicted with either Familial Hypertrophic or Dilated Cardiomyopathy. In addition, mutations in the skeletal isoforms of MyBP-C are associated with distal arthrogryposis (i.e. club foot). Cardiac MyBP-C Isoform: Since the heart muscle generates the power needed to support blood flow through the vascular system, altered power production may result from mutations to cMyBP-C. These MyBP-C mutations may compromise its ability to modulate the actomyosin motor’s power generation. Therefore, the power generated by native cardiac myosin thick filaments isolated from transgenic mouse models (in collaboration with the Robbins Lab) containing mutant MyBP-C are assessed in the laser trap assay. In addition, MyBP-C’s structure and its function are modulated by phosphorylation of several serines in the N terminus, the mechanism by which is a target for pharmaceutical intervention. These studies, in collaboration with both the Craig Lab and Previs Lab, will define the relationship between MyBP-C’s structure and its ability to interact with myosin and/or actin at the molecular level in the hopes of providing a molecular basis for genetic forms of heart failure. Skeletal MyBP-C Isoforms: With 3 isoforms of MyBP-C being differentially expressed in cardiac, slow skeletal, and fast skeletal muscles, are the structural differences that differentiate these isoforms a key to potential functional differences? If so, are their functional capacities matched to the physiological demands of the muscles in which they are expressed? We take advantage of the naturally occurring isoforms by using molecular biophysics in a comparative approach to address these questions in collaboration with the Craig Lab and Sadayappan Lab. |
|
  |
