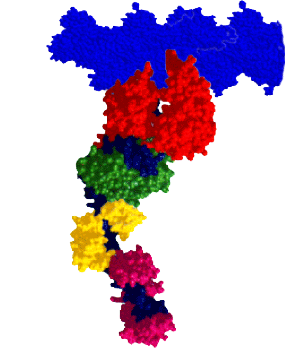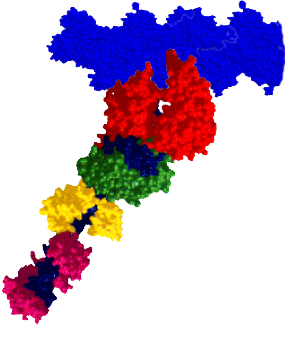
RESEARCH INTERESTSMyosin: A Molecular MotorMyosin is a molecular motor that steps along actin tracks. This motor’s ability to generate force and motion relies on its capacity to hydrolyze ATP and to convert chemical energy into mechanical work. The myosin superfamily now consists of nearly 30 different classes of myosin associated with biological functions as diverse as muscle contraction, organelle transport, cell motility, and cell division. Our laboratory and collaborators focus on an extremely diverse group of myosins: Class II: Skeletal, smooth, and cardiac myosins responsible for muscle contraction. Class V: A long-necked processive myosin associated with vesicular transport directed towards the cell periphery (collaborators: Trybus Lab, Walcott Lab). Class XIV: A single-headed myosin involved in Toxoplasma gondii parasite locomotion and host cell invasion (collaborator: Ward Lab). Class XXVII: A long-necked myosin, similar to the Class V myosins, involved in dense granule transport in Toxoplasma gondii (collaborator: Heaslip Lab). These myosins share significant structural and functional capacities, i.e. they possess a catalytic domain that has hydrolytic, actin-binding, and motor capacities. Emerging from the motor domain is a light-chain and/or calmodulin-binding domain that serves as a mechanical lever to amplify small conformational changes that originate within the motor domain’s active site. The length of this lever can vary depending on the myosin class. The differences in both structure and function among the various myosin classes can provide a model system to help probe the molecular structure and function of myosin as a chemomechanical enzyme. |
Warshaw Laboratory – University of Vermont