|
RESEARCH INTERESTS
Myosin Binding Protein-C (MyBP-C)
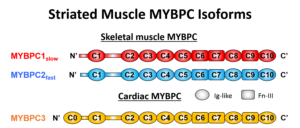
MyBP-C is a striated muscle contractile protein, mutations to which have been linked to genetic forms of hypertrophic and dilated cardiomyopathies as well as skeletal muscle myopathies (i.e. distal arthrogryposis). MyBP-C is expressed as 3 isoforms; fast skeletal, slow skeletal, and cardiac. It’s an elongated protein with at least 10 globular domains (yellow structures). MyBP-C associates with the myosin thick filament at one end and both the myosin head region and actin at its other end.
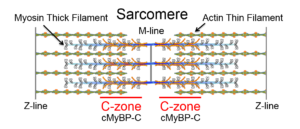
 Striated muscles are composed of repeating contractile units called sarcomeres in which the thin, actin-containing and thick, myosin-containing filaments overlap and slide past each other during muscle shortening. The myosin thick filament is composed of 300 myosin molecules that form a bipolar filament in which the myosin heads on each half of the thick filament are oriented to move the thin filament towards the center of the sarcomere. Interestingly, MyBP-C is localized to two regions (C-zones) near the center of the thick filament and thus the sarcomere. Why is MyBP-C localized to only these discrete zones is a major lab interest? Striated muscles are composed of repeating contractile units called sarcomeres in which the thin, actin-containing and thick, myosin-containing filaments overlap and slide past each other during muscle shortening. The myosin thick filament is composed of 300 myosin molecules that form a bipolar filament in which the myosin heads on each half of the thick filament are oriented to move the thin filament towards the center of the sarcomere. Interestingly, MyBP-C is localized to two regions (C-zones) near the center of the thick filament and thus the sarcomere. Why is MyBP-C localized to only these discrete zones is a major lab interest?
 Although MyBP-C affects actomyosin function, the molecular mechanism by which this occurs is far from certain, with multiple models proposed for its action. The specific model depends on MyBP-C’s binding partner. For example, by binding to the myosin head region, MyBP-C may limit myosin’s interaction with actin and maintain myosin in a super-relaxed state. Alternatively, MyBP-C may bind to actin and act as an internal load to shortening or activate the thin filament in a calcium-independent manner. Although MyBP-C affects actomyosin function, the molecular mechanism by which this occurs is far from certain, with multiple models proposed for its action. The specific model depends on MyBP-C’s binding partner. For example, by binding to the myosin head region, MyBP-C may limit myosin’s interaction with actin and maintain myosin in a super-relaxed state. Alternatively, MyBP-C may bind to actin and act as an internal load to shortening or activate the thin filament in a calcium-independent manner.
 Our laboratory uses molecular biophysics to unravel the key interactions between MyBP-C and both actin and myosin that allow this unique protein to modulate actomyosin function in the heart and in skeletal muscles. Our laboratory uses molecular biophysics to unravel the key interactions between MyBP-C and both actin and myosin that allow this unique protein to modulate actomyosin function in the heart and in skeletal muscles.
|

