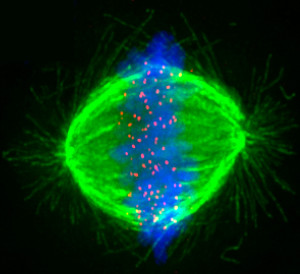
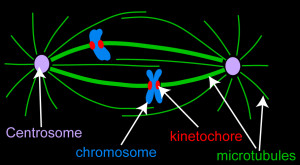
 The Stumpff Lab uses advanced microscopy techniques to investigate the mechanisms that move and organize chromosomes during cell division and determine how these functions preserve genomic integrity. Abnormal control of chromosome movements leads to chromosome segregation errors and the production of aneuploid cells, i.e. cells with the wrong number of chromosomes. Aneuploidy is implicated in the initiation and development of cancer, is a leading cause of human miscarriages, and underlies monosomy and trisomy syndromes (e.g. Turner’s syndrome, Down’s syndrome, Edward’s syndrome and Patau syndrome). Thus, elucidating the mechanisms that control chromosome movements during cell division is an important step towards understanding the molecular basis of a wide range of human health disorders and identifying novel treatment targets.
The Stumpff Lab uses advanced microscopy techniques to investigate the mechanisms that move and organize chromosomes during cell division and determine how these functions preserve genomic integrity. Abnormal control of chromosome movements leads to chromosome segregation errors and the production of aneuploid cells, i.e. cells with the wrong number of chromosomes. Aneuploidy is implicated in the initiation and development of cancer, is a leading cause of human miscarriages, and underlies monosomy and trisomy syndromes (e.g. Turner’s syndrome, Down’s syndrome, Edward’s syndrome and Patau syndrome). Thus, elucidating the mechanisms that control chromosome movements during cell division is an important step towards understanding the molecular basis of a wide range of human health disorders and identifying novel treatment targets.
We are currently investigating the molecular control of mitotic chromosome alignment and how it impacts chromosome structure and organization. The alignment of mitotic chromosomes at the spindle equator prior to their division is widely conserved among eukaryotes, suggesting the process plays an essential function. However, the mechanisms that control chromosome movements to facilitate their alignment and how these mechanisms contribute to cellular fitness are not understood. Our studies implicate key roles for several molecular motor proteins in the mechanical control of chromosome movements and the preservation of genomic integrity. We are addressing fundamental questions aimed at understanding the molecular basis and downstream impact of these mechanical controls.
CURRENT PROJECTS: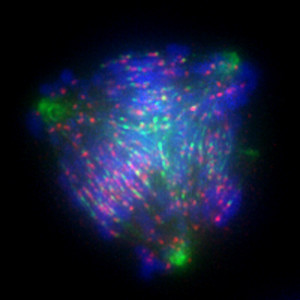
1. How do molecular motor proteins contribute to the spatial and temporal control of chromosome movements within the mitotic spindle?
2. How does the spatial organization of mitotic chromosome movements impact the accurate segregation of the genome and its 3D organization during interphase?
3. Can proteins involved in controlling mitotic chromosome movements be exploited as anti-cancer targets?